
Based on the know-how we have accumulated through the development and manufacture of cultured corneal epithelial cell sheet for ophthalmologic use and bone formation materials for dental treatment, ArBlast is providing customers with Cell Processing System, which conforms to GMP.
Thus far, it has been difficult for university labs to upgrade their manufacturing control and quality control to an extent that meets the level required by GMP, since such labs cannot afford large expenditures on equipment and facilities
Using the know-how we have accumulated through the development of cell processing systems at our own cell processing center (CPC) and various other research institutes and laboratories, we provide both the hardware and software of a reliable, integrated cell processing system.
We would be greatly pleased if you found our cell processing system useful for your research and development activities.

1,Best Environment
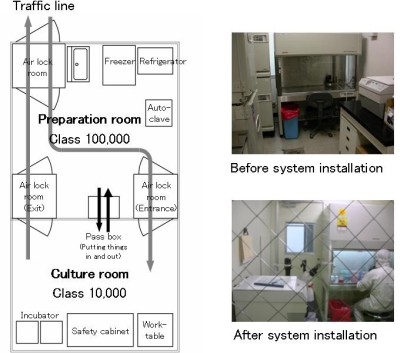
CPS is designed to keep operating room pressure positive and cleanliness level at 10,000.
2,Flexible Layout
CPS is tailored to fit the structure of the building in which it is installed.
3,Low Cost
Existing air-conditioning equipment and other machinery can be placed inside the CPC facility.

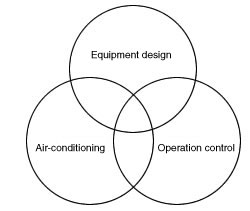 There are 3 elements essential for the cell-processing quality control system: equipment design, air-conditioning and operation control. There are 3 elements essential for the cell-processing quality control system: equipment design, air-conditioning and operation control.
ArBlast provides integrated assistance in establishing a cell processing system that contains these 3 vital elements.
Equipment design
Traffic lines designed to prevent cross contamination and intermixing. An area for aseptic treatment distinctly separate from the other areas.
Air-conditioning
Room pressure, temperature, humidity and cleanliness level properly controlled. Distinct classification of cleanliness levels.
Operation control
Implementation of regular validation. Training programs, work records and operation records to prevent human error.

Addition of equipment (preparation room, pass box, air shower etc.)
Conclusion of agreement to provide maintenance service (cleanliness level control, hepafilter change etc.) after operation commencement
Operation support (preparation of GMP control manual)

|